The Standard Model of Particle Physics
Man has always strived to better understand the world that we live in. One particularly vexing question has always been: what exactly makes up the universe? Is our universe made up of an infinite number of differing objects, or is there a set of fundamental building blocks that combine in different ways, therefore composing everything in the universe?Over two thousand years ago, Aristotle determined that the world was made up of earth, wind, fire and air. Since Aristotle, Greek philosophers proposed the idea that atoms – even though they could not be seen or felt – were a set of miniscule, indestructible balls of which everything was composed. By the late 1800’s it was determined based on behavior that atoms must have an internal structure, therefore they could not be fundamental.
Think about that for a moment. People were proposing that these certain fundamental particles existed, even though they had no way of proving it! It is that very same process that has shaped our current (and ever-changing) view of the universe. This has been guided by a concept known as the The Standard Model of Physics. The Standard Model is a scientific theory that attempts to unify the fundamental structure of matter and forces acting upon it into a small number of basic entities.
Fundamental forces? Yes, they are out there. For example, you know that gravity is very real. If you were to knock your pencil off of your desk, each and every time it would fall towards the ground. Gravity is a force, no one knows for sure what causes gravity, but we all agree that it exists! Remember that the next time you trip and fall down!
By the 1930’s the atomic model described the atom as consisting of a nucleus made up of protons and neutrons, which in turn are orbited by electrons. Also included in this original atomic model was a particle with no mass called a gamma particle (photon). Different combinations of these four elementary particles seemed to concisely describe all of the matter of the known universe. However, as time went on and technology developed, there soon emerged radioactive decay studies that upset this simple picture of atomic structure.
| In radioactive decay, alpha particles and gamma rays emitted by radioactive nuclei have single energies that depend on the decaying nucleus from which they originate. However, beta particles are emitted with a wide range of energies. It was then postulated that an unseen neutral particle was emitted with the beta particles and was named a “neutrino” by Enrico Fermi, a pioneer in this field of study. Between the 1930’s and the 1960’s more particles where being identified including the the positron, the muon and the pion. Particle accelerator experiments resulted in the identification of more and more particles and it became obvious that these were not able to be considered elementary particles but they were, in fact, made up of other particles… known as quarks. Quarks have fractional charges, (i.e., +2/3 or -1/3, and so forth) but even with a fractional charge, all of the particles they make will have a whole number charge. | 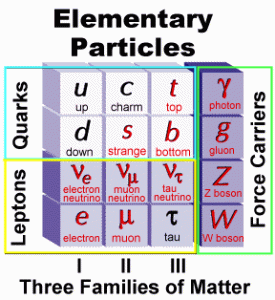 |
